Organ Donation and Transplant Oversight Structure
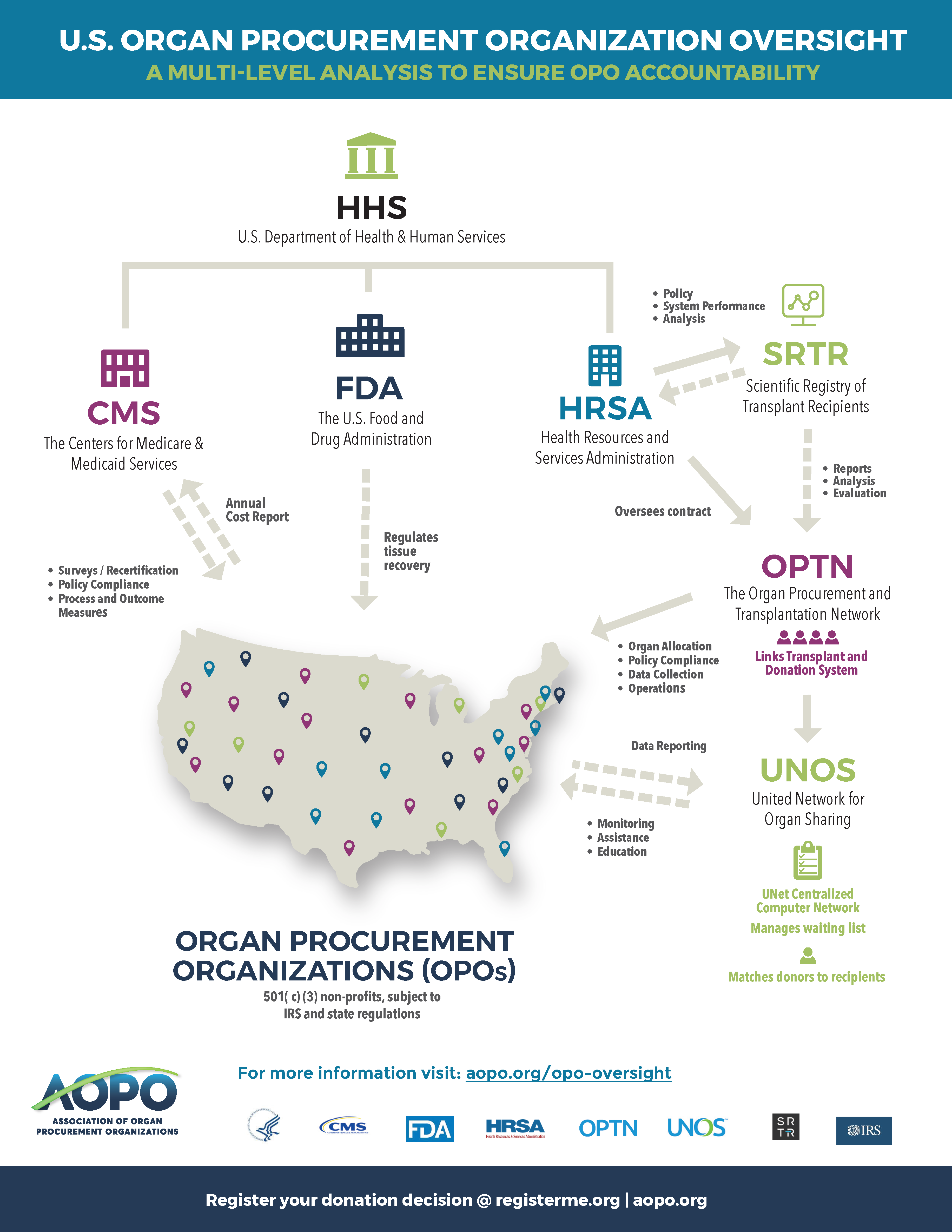
The organ donation and transplant ecology are complex. The National Organ Transplant Act (NOTA) of 1984 established the Organ Procurement and Transplantation Network (OPTN) to create and define organ allocation and distribution policies. The OPTN operates under a federal contract that is regulated and overseen by the Department of Health and Human Services’ Health Resources and Services Administration (HRSA) and managed by the United Network for Organ Sharing (UNOS), a private, non-profit organization created to run the OPTN and transplant waiting list.
The OPTN shares responsibility with the Centers for Medicare & Medicaid Services (CMS) for overseeing Organ Procurement Organizations (OPOs) and transplant centers. By law, OPOs must be nonprofit entities and members of the OPTN. Transplant Centers must also be OPTN members. There are currently 57 OPOs responsible for identifying eligible donors and recovering organs from deceased donors in the United States, with no current statutory authority to add new OPOs. Each OPO is assigned a geographic donation service area (DSA).
Transplant centers and OPOs are among the 20 healthcare organizations regulated by CMS Conditions of Participation (CoPs) and Conditions for Coverage (CfCs) requirements. As part of these, transplant centers and OPOs must meet specified CMS performance standards, or risk losing their CMS certification or DSA. CMS relies on analyses by the Scientific Registry of Transplant Recipients (SRTR) to evaluate OPO and transplant center performance, which is compiled based on OPTN data.